- Treatment
Landscape- ABSSSI
Treatment
Burden- ABSSSI
Treatment
Challenges- About
KIMYRSA®- What is
KIMYRSA®?- Mechanisms
of Action- PK Profile
- In Vitro
Results by Pathogen- Clinical
Studies- Clinical Study Information
- SOLO Studies
- Real-World
Experience- Real-World Studies
- Real Patient Results
- Single-Dose
Administration- Resources
and Support

ORBACTIV® (oritavancin) was studied in 2 large, identically designed, phase 3 trials1,2
The efficacy of KIMYRSA® has been established from adequate and well-controlled trials of another oritavancin product, ORBACTIV®, in patients with ABSSSI.
SOLO I and SOLO II1,2
- Two global, multicenter, randomized, double-blind studies
- Designed to compare the efficacy, safety, and noninferiority of single-dose intravenous ORBACTIV® (oritavancin) vs intravenous vancomycin for 7 to 10 days in adults with ABSSSIs (including cellulitis, abscesses, and wound infections)
- 1,959 study participants (oritavancin, 978; vancomycin, 981)
SOLO I: A global, randomized, double-blind study.1
SOLO II: A global, multicenter, randomized, double-blind, comparative efficacy and safety study2Study Design for SOLO I and SOLO II
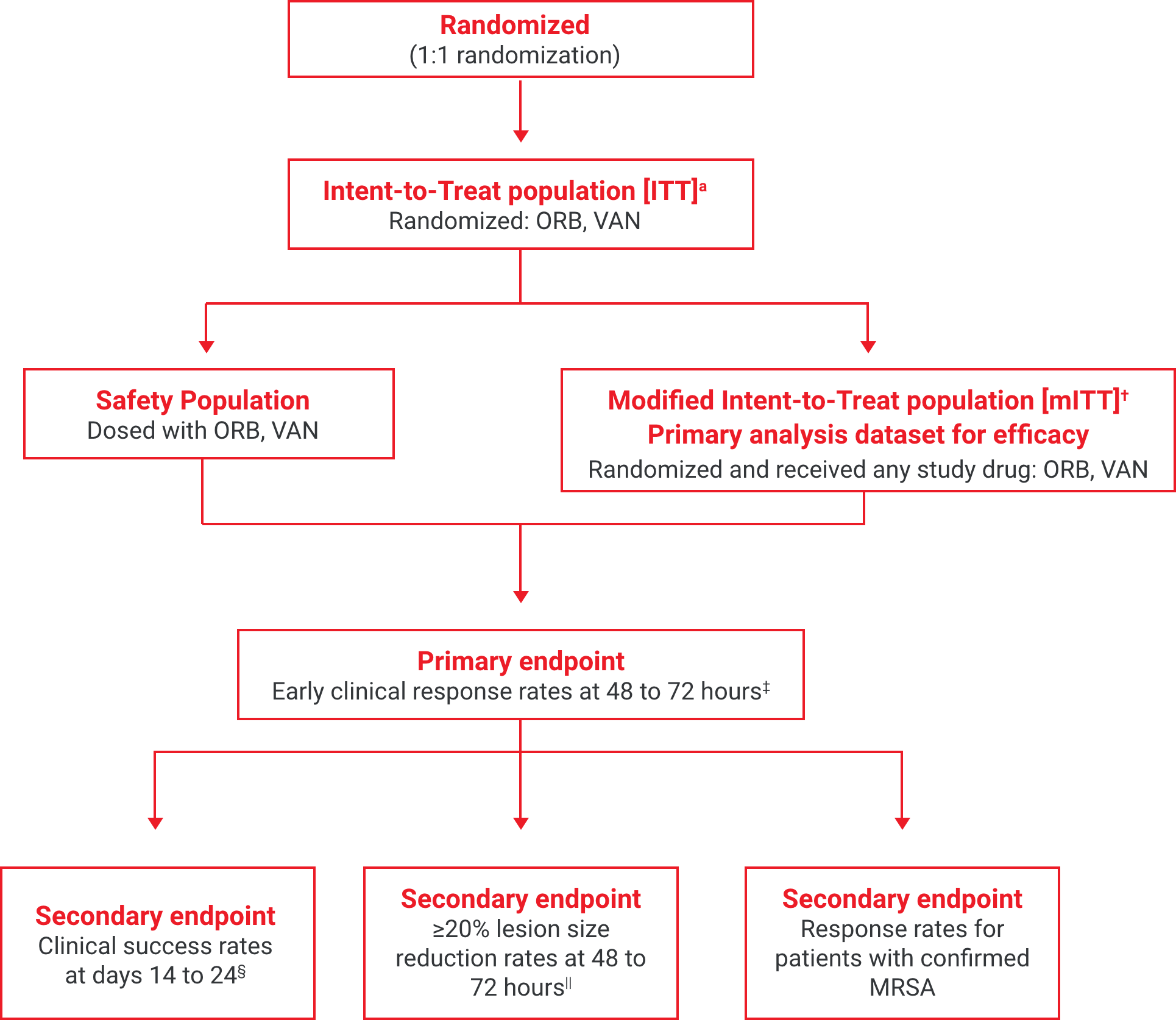
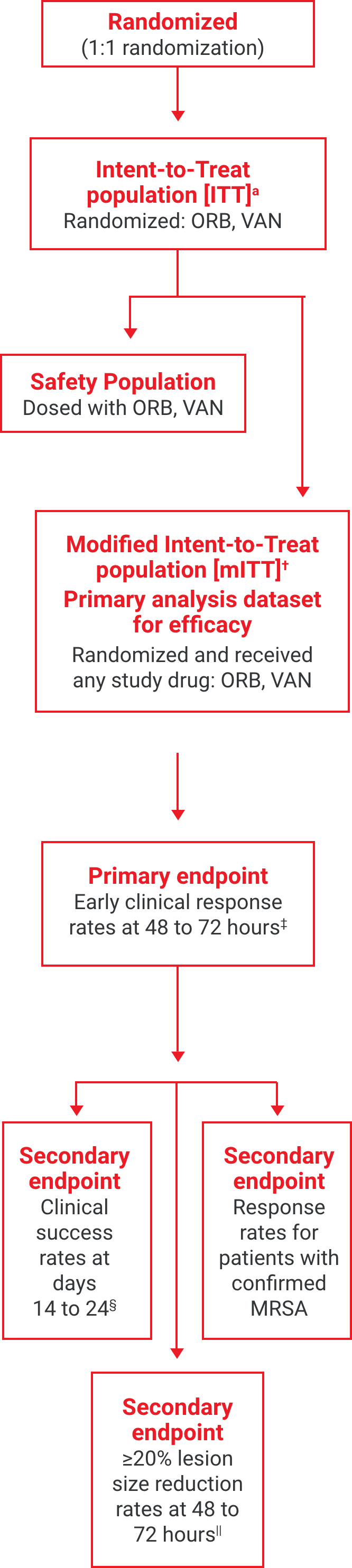
aThe intent-to-treat (ITT) population included all patients randomized into the study.2
†The modified intent-to-treat (mITT) population was the primary population for all the efficacy analyses and included all randomized patients who received any study drug.2
‡Early clinical response defined as a composite of the cessation of spread or reduction in size of baseline lesion, absence of fever, and no rescue antibacterial drug at 48 to 72 hours.1
§Investigator-assessed clinical success at days 14 to 24, defined as complete or nearly complete resolution of baseline signs and symptoms related to primary ABSSSI site such that no further treatment with antibacterial drugs was needed.3
||Patients achieving a ≥20% reduction in lesion area from baseline at 48 to 72 hours after initiation of therapy.1,2
SOLO I and SOLO II demographic baseline characteristics of the study participants (mITT population)1,2¶#**††

Baseline characteristics
SOLO I & II ORBACTIV® (n=978)
SOLO I & II vancomycin (n=981)
Age, y, range 18-89 18-93 Male sex, n (%) 639 (65.3) 644 (65.6) Race, n(%)** - White
- Black
- Asian
- Other
630 (64.4)
57 (5.8)
275 (28.1)
16 (1.6)
631 (64.3)
57 (5.8)
275 (28.1)
17 (1.7)Body weight, median, kg 75.2 76.0 Infection type - Wound, n (%)
- Confirmed MRSA, n/N (%)
- Cellulitis, n (%)
- Confirmed MRSA, n/N (%)
- Abscess, n (%)
- Confirmed MRSA, n/N (%)
283 (28.9)
62/295 (21)
387 (39.6)
32/248 (12.9)
308 (31.5)
110/272 (40.4)
281 (28.6)
65/276 (23.6)
400 (40.8)
41/267 (15.4)
300 (30.6)
95/259 (36.7)Diabetes mellitus, n (%) 139 (14.2) 140 (14.3) Lesion area, median, cm 267.9 267.2 Any antibiotic prior to trial drug 102 181 Positive infection-site culture - Any gram-positive pathogen, n/N (%)
- S. aureus
- MRSA, n††
619/641 (96.6)
468/619 (75.6)
204
621/642 (96.7)
468/621 (75.4)
201¶mITT population: all randomized patients who received any study drug.1
#There were no significant between-group differences except for age (P=0.04, calculated with the use of the Kruskal–Wallis test) and a positive blood culture for S. aureus (P=0.002, calculated with the use of Fisher’s exact test). Percentages may not add up to 100 because of rounding.
**Race was self-reported.
††Includes both infection site culture and blood culture.
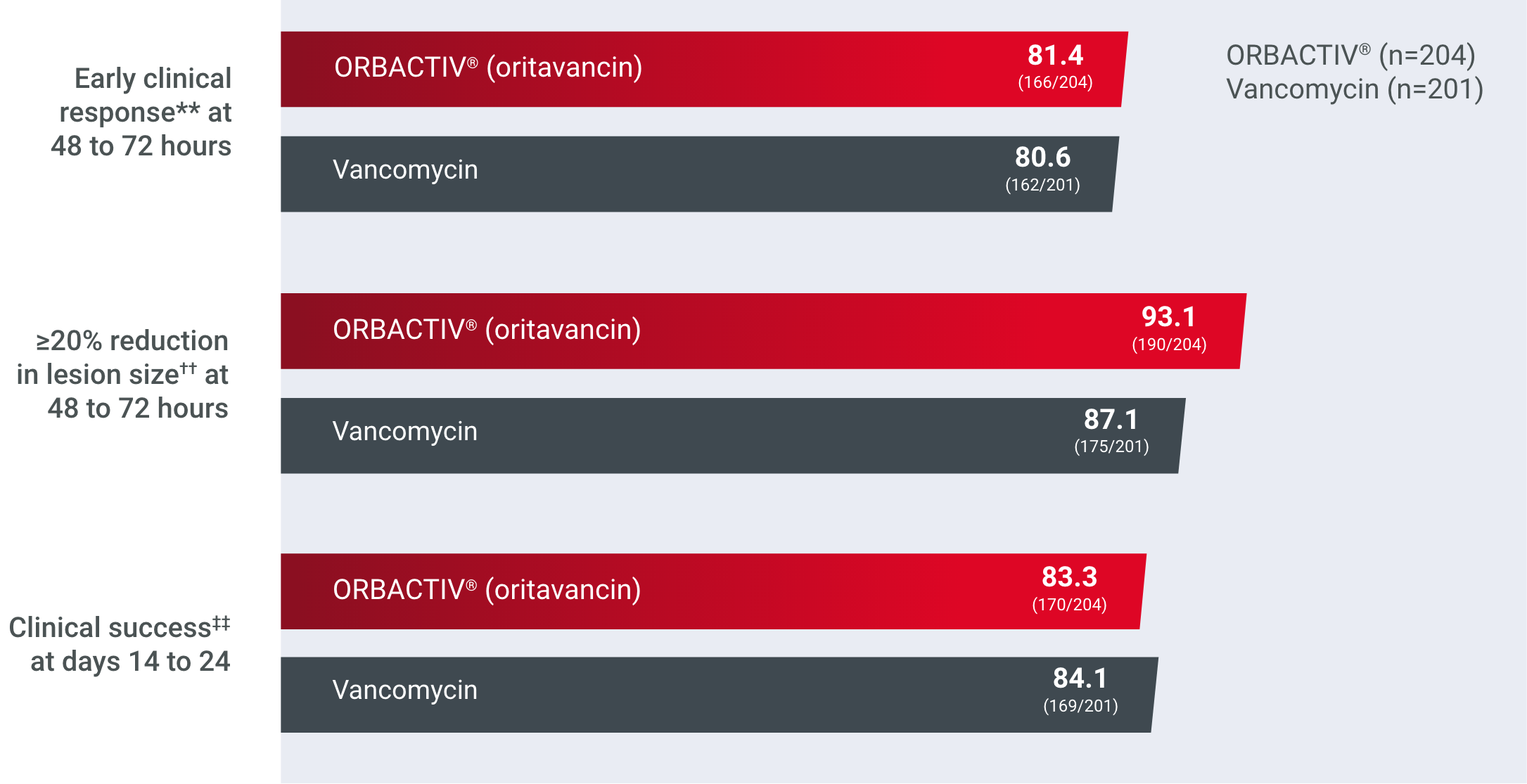
Single-dose ORBACTIV® achieved early clinical response at 48 to 72 hours in adults with ABSSSIs, including MRSA1
ABSSSI, acute bacterial skin and skin structure infection; max, maximum; min, minimum; MRSA, methicillin-resistant Staphylococcus aureus.
References: 1. Corey GR, Kabler H, Mehra P, et al. Single-dose oritavancin in the treatment of acute bacterial skin infections. N Engl J Med. 2014;370(23):2180-2190. doi:10.1056/NEJMoa1310422 2. Corey GR, Good S, Jiang H, et al. Single-dose oritavancin versus 7-10 days of vancomycin in the treatment of gram-positive acute bacterial skin and skin structure infections: the SOLO II noninferiority study. Clin Infect Dis. 2015;60(2):254-262. doi:10.1093/cid/ciu778 3. Kimyrsa. Package insert. Melinta Therapeutics, LLC; 2021.
The efficacy of KIMYRSA® has been established from adequate and well-controlled trials of another oritavancin product, ORBACTIV®, in patients with ABSSSI.
Primary endpoint: early clinical response rates at 48 to 72 hours1,2a
Comparator, % (n/N), mITT population†

Study design: Two global, multicenter, randomized, double-blind studies designed to compare the efficacy, safety, and noninferiority of single-dose intravenous ORBACTIV® (oritavancin) vs intravenous vancomycin for 7 to 10 days in adults with ABSSSIs (including cellulitis, abscesses, and wound infections).1,2
aEarly clinical response defined as a composite of the cessation of spread or reduction in size of baseline lesion, absence of fever, and no rescue antibacterial drug at 48 to 72 hours.1,2
†mITT population: all randomized patients who received any study drug.1,2
Reduced lesion sizes in patients with ABSSSI1,2‡
Secondary endpoint: ≥20% reduction in lesion size at 48 to 72 hours1,2‡
Comparator, % (n/N), mITT population§

Study design: Two global, multicenter, randomized, double-blind studies designed to compare the efficacy, safety, and noninferiority of single-dose intravenous ORBACTIV® (oritavancin) vs intravenous vancomycin for 7 to 10 days in adults with ABSSSIs (including cellulitis, abscesses, and wound infections).1,2
‡Patients achieving a ≥20% reduction in lesion area from baseline at 48 to 72 hours after initiation of therapy.1,2
§mITT population: all randomized patients who received any study drug.1,2
High cure rates of ABSSSI1,2||
Secondary endpoint: clinical success rates at days 14 to 241,2||
Comparator, % (n/N), mITT population¶

Study design: Two global, multicenter, randomized, double-blind studies designed to compare the efficacy, safety, and noninferiority of single-dose intravenous ORBACTIV® (oritavancin) vs intravenous vancomycin for 7 to 10 days in adults with ABSSSIs (including cellulitis, abscesses, and wound infections).1,2
||Investigator-assessed clinical success at days 14 to 24, defined as complete or nearly complete resolution of baseline signs and symptoms related to primary ABSSSI site such that no further treatment with antibacterial drugs was needed.1,2
¶mITT population: all randomized patients who received any study drug.1,2
Demonstrated efficacy in the largest MRSA subset of any study of long-acting ABSSSI therapies3
Trends indicate an increase in the incidence of MRSA in 2019-2020.4
Response rates for patients with confirmed MRSA in pooled SOLO trials3
Comparator, % (n/N), microITT population#
Study design: Two global, multicenter, randomized, double-blind studies designed to compare the efficacy, safety, and noninferiority of single-dose intravenous ORBACTIV® (oritavancin) vs intravenous vancomycin for 7 to 10 days in adults with ABSSSIs (including cellulitis, abscesses, and wound infections).1,2
#The microITT population consisted of all mITT patients with baseline gram-positive pathogen(s) known to cause ABSSSI and they were used for the secondary efficacy analyses.1,2
**Early clinical response defined as a composite of the cessation of spread or reductions in size of baseline lesion, absence of fever, and no rescue antibacterial drug at 48 to 72 hours.1,2
††Patients achieving a ≥20% reduction of lesion area from baseline at 48 to 72 hours.1,2
‡‡Investigator-assessed clinical success at days 14 to 24, defined as complete or nearly complete resolution of baseline signs and symptoms related to the primary ABSSSI site such that no further treatment with antibacterial drugs was needed.1,2
A well-established safety profile
ABSSSI, acute bacterial skin and skin structure infection; microITT, microbiological intent-to-treat; mITT, modified intent-to-treat; MRSA, methicillin-resistant Staphylococcus aureus.
References: 1. Corey GR, Kabler H, Mehra P, et al. Single-dose oritavancin in the treatment of acute bacterial skin infections. N Engl J Med. 2014;370(23):2180-2190. doi:10.1056/ NEJMoa1310422 2. Corey GR, Good S, Jiang H, et al. Single-dose oritavancin versus 7-10 days of vancomycin in the treatment of gram-positive acute bacterial skin and skin structure infections: the SOLO II noninferiority study. Clin Infect Dis. 2015;60(2):254-262. doi:10.1093/cid/ciu778 3. Kimyrsa. Package insert. Melinta Therapeutics; 2021. 4. SENTRY Antimicrobial Surveillance Program. JMI MVP. Accessed July 1, 2021. https://sentry-mvp.jmilabs.com
The safety of KIMYRSA® has been established from adequate and well-controlled trials of another oritavancin product, ORBACTIV®and in a PK study of KIMYRSA® in patients with ABSSSI.
Most commonly reported adverse reactions in the pooled ABSSSI clinical trials1a

Pooled SOLO trials, adverse reactions
ORBACTIV®
(N=976), n (%)Vancomycin
(N=983), n (%)Gastrointestinal disorders Diarrhea 36 (3.7) 32 (3.4) Nausea 97 (9.9) 103 (10.5) Vomiting 45 (4.6) 46 (4.7) Nervous system disorders Dizziness 26 (2.7) 26 (2.9) Headaches 69 (7.1) 66 (6.7) General disorders and administration Infusion site phlebitis 24 (2.5) 15 (1.5) Infusion site reaction 19 (1.9) 34 (3.5) Infections and infestations Abscess (limb and subcutaneous) 37 (3.8) 23 (2.3) Investigations Alanine aminotransferase increased 27 (2.8) 15 (1.5) Aspartate aminotransferase increased 18 (1.8) 15 (1.5) Cardiac disorders Tachycardia 24 (2.5) 11 (1.1) a≥1.5% of patients receiving ORBACTIV®.- Safety population (N=1959); all patients who received the assigned study drugs1
- The most commonly reported serious adverse reaction was cellulitis in both treatment groups (ORBACTIV®, 1.1%; vancomycin, 1.2%)1
Safety demonstrated in the KIMYRSA® PK trial
Summary of TEAEs by system organ class and preferred term (safety analysis set)2†‡§||

System organ class preferred term
ORBACTIV®
(3-hour IV, n=52)KIMYRSA®
(1-hour IV, n=50)Patients with at least 1 TEAE, n (%) 31 (59.6) 24 (48.0) TEAEs in ≥5% of patients Gastrointestinal disorders, n (%) Nausea 3 (5.8) 1 (2.0) Diarrhea 5 (9.6) 3 (6.0) General disorders and administration site conditions, n (%) Chills 1 (1.9) 3 (6.0) Pyrexia 1 (1.9) 3 (6.0) †Events occurring in ≥5% of patients.
‡Safety demonstrated in an open-label, multicenter, PK study that compared KIMYRSA® administered over 1 hour (n=50) with ORBACTIV® administered over 3 hours (n=52) for the treatment of adult patients with ABSSSI.2
§Safety data were collected up to 60 days following ORBACTIV® treatment.3
||Safety analysis set included all subjects who received any amount of IV oritavancin.3
Results observed in the higher-risk patients such as those you see in clinical practice3
ABSSSI, acute bacterial skin and skin structure infection; PK, pharmacokinetic; TEAE, treatment-emergent adverse event.
References: 1. Orbactiv. Package insert. Melinta Therapeutics; 2019. 2. Data on file. Melinta Therapeutics. 3. Redell M, Moeck G, Lucasti C, et al. A real-world patient registry for oritavancin demonstrates efficacy and safety consistent with the phase 3 SOLO program. Open Forum Infect Dis. 2018;5(6):ofy051. doi:10.1093/ofid/ofy051
*INDICATION AND USAGE
- Both KIMYRSA® and ORBACTIV® are oritavancin products that are indicated for the treatment of adult patients with acute bacterial skin and skin structure infections (ABSSSI) caused or suspected to be caused by susceptible isolates of the following gram-positive microorganisms: Staphylococcus aureus (including methicillin-susceptible [MSSA] and methicillin-resistant [MRSA] isolates), Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus dysgalactiae, Streptococcus anginosus group (includes S. anginosus, S. intermedius, and S. constellatus), and Enterococcus faecalis (vancomycin-susceptible isolates only).
- To reduce the development of drug-resistant bacteria and maintain the effectiveness of oritavancin and other antibacterial drugs, oritavancin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria.
- KIMYRSA® and ORBACTIV® are not approved for combination use and have differences in dose strength, duration of infusion, and preparation instructions, including reconstitution and dilution instructions and compatible diluents. Please see the full Prescribing Information for each product.
IMPORTANT SAFETY INFORMATION
Contraindications
- Use of intravenous unfractionated heparin sodium is contraindicated for 120 hours (5 days) after oritavancin administration because the activated partial thromboplastin time (aPTT) test results may remain falsely elevated for approximately 120 hours (5 days) after oritavancin administration.
- Oritavancin products are contraindicated in patients with known hypersensitivity to oritavancin.
Warnings and Precautions
- Coagulation test interference: Oritavancin has been shown to artificially prolong aPTT for up to 120 hours, and may prolong PT and INR for up to 12 hours and ACT for up to 24 hours. Oritavancin has also been shown to elevate D-dimer concentrations up to 72 hours. For patients who require aPTT monitoring within 120 hours of oritavancin dosing, consider a non-phospholipid dependent coagulation test such as a Factor Xa (chromogenic) assay or an alternative anticoagulant not requiring aPTT.
- Serious hypersensitivity reactions, including anaphylaxis, have been reported with the use of oritavancin products. Discontinue infusion if signs of acute hypersensitivity occur. Closely monitor patients with known hypersensitivity to glycopeptides.
- Infusion related reactions: Infusion reactions characterized by chest pain, back pain, chills and tremor have been observed with the use of oritavancin products, including after the administration of more than one dose of oritavancin during a single course of therapy. Stopping or slowing the infusion may result in cessation of these reactions.
- Clostridioides difficile-associated diarrhea: Evaluate patients if diarrhea occurs.
- Concomitant warfarin use: Oritavancin has been shown to artificially prolong PT/INR for up to 12 hours. Patients should be monitored for bleeding if concomitantly receiving oritavancin products and warfarin.
- Osteomyelitis: Institute appropriate alternate antibacterial therapy in patients with confirmed or suspected osteomyelitis.
- Prescribing oritavancin products in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of development of drug-resistant bacteria.
Adverse Reactions
- The most common adverse reactions (≥3%) in patients treated with oritavancin products were headache, nausea, vomiting, limb and subcutaneous abscesses, and diarrhea. The adverse reactions occurring in ≥2 patients receiving KIMYRSA® were hypersensitivity, pruritus, chills and pyrexia.
Please see Full Prescribing Information for ORBACTIV®.
Please see Full Prescribing Information for KIMYRSA®.
SEE MORE
*INDICATION AND USAGE
Both KIMYRSA® and ORBACTIV® are oritavancin products that are indicated for the treatment of adult patients with acute bacterial skin and skin structure infections (ABSSSI) caused or suspected to be
IMPORTANT SAFETY INFORMATION
ContraindicationsUse of intravenous unfractionated heparin sodium is contraindicated for 120 hours (5 days) after oritavancin
- Real Patient Results
- SOLO Studies
- Mechanisms
- ABSSSI
- ABSSSI
This site is intended for US Healthcare Professionals only.


