- Treatment
Landscape- ABSSSI
Treatment
Burden- ABSSSI
Treatment
Challenges- About
KIMYRSA®- What is
KIMYRSA®?- Mechanisms
of Action- PK Profile
- In Vitro
Results by Pathogen- Clinical
Studies- Clinical Study Information
- SOLO Studies
- Real-World
Experience- Real-World Studies
- Real Patient Results
- Clinical Expert Videos
- Single-Dose
Administration- Resources
and Support

Skin and soft tissue infections (SSTI) place a significant burden on the health care system1
ABSSSI-related hospitalizations and readmissions continue to be a significant burden2
 Nearly twice as many ED visits for SSTI in 2015 vs 2000.3
Nearly twice as many ED visits for SSTI in 2015 vs 2000.3Based on 2017 and 2018 HCUP data
In 2017, there were 3.3 million ED visits1
In 2018, there were 529,590 total hospitalization admissions4
In a 2018 study of data collected from 2012 to 2013, 12.1% of patients with ABSSSI had an unplanned skin infection–related ED visit or readmission within 30 days post discharge5
Admissions for ABSSSI impose a substantial economic burden on US hospitals, with more than half of that burden accounted for by room costs6
ABSSSI-Related Hospital Costs
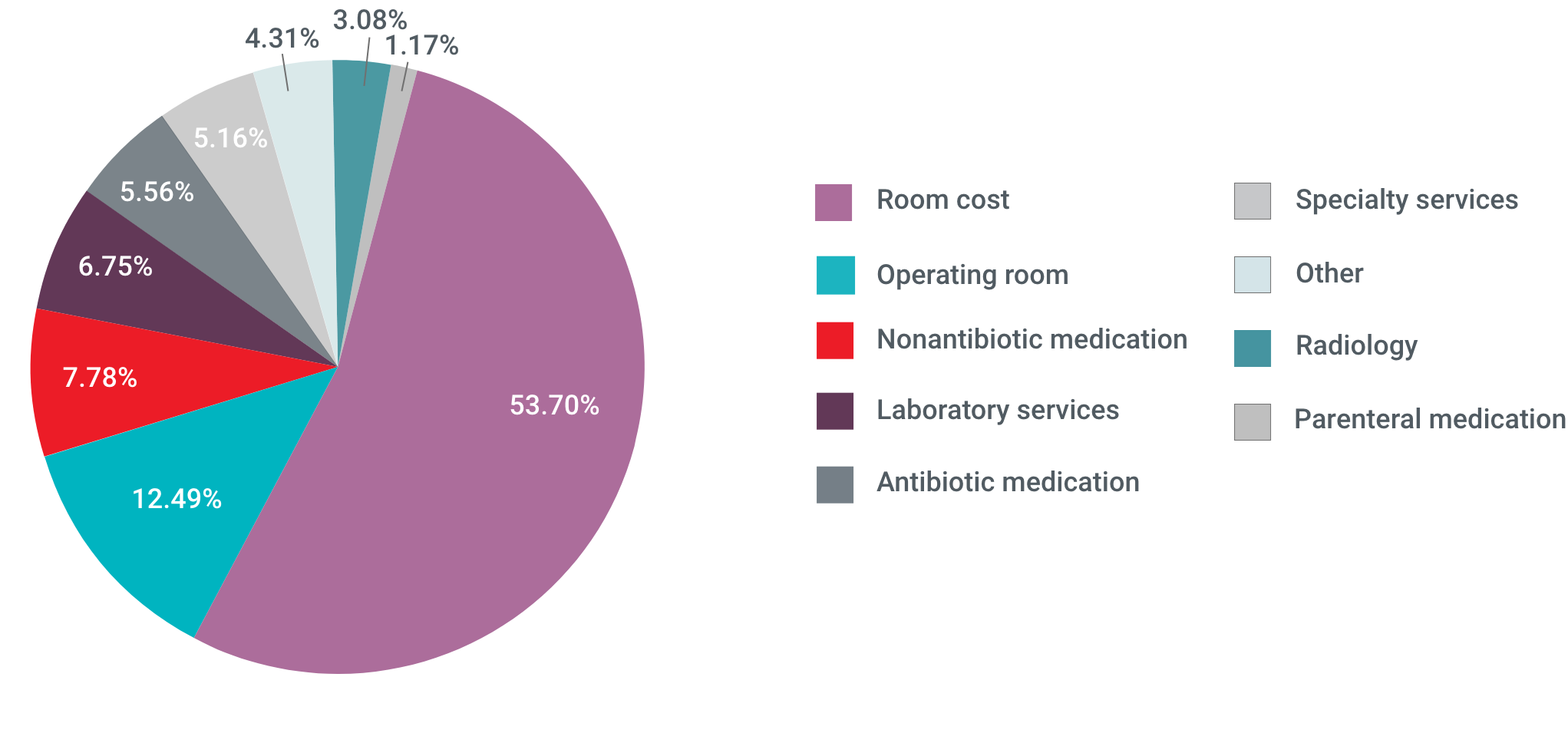
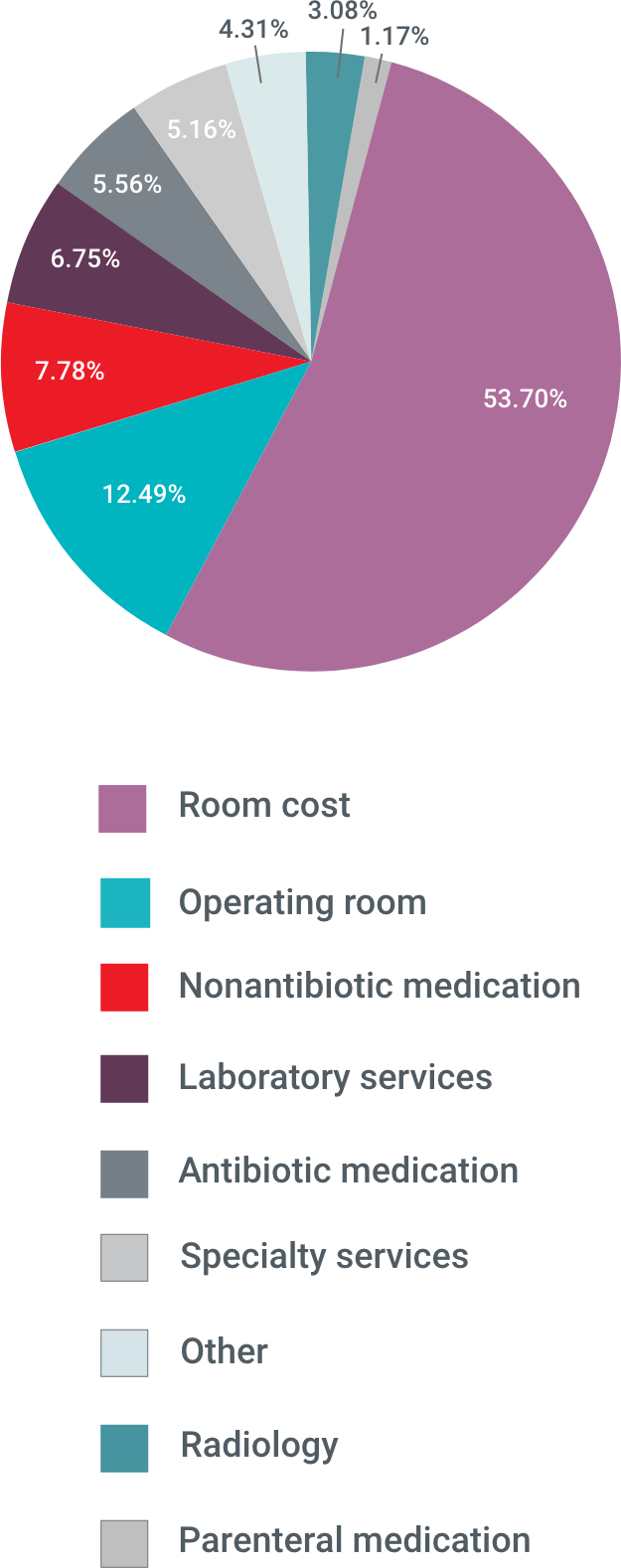
Based on data collected between 2007 and 2009, aggregate hospital costs were $6 billion annually7
In 2018, patients with skin and subcutaneous tissue infections had a mean LOS of 4.0 days4
The 2017 HCUP ranks SSTI the 8th most common reason for ED visits resulting in admission to the hospital.1
There’s a different approach to treatment
ABSSSI, acute bacterial skin and skin structure infection; ED, emergency department; HCUP, Healthcare Cost and Utilization Project.
References: 1. Agency for Healthcare Research and Quality. 2017 Data from HCUPnet, Healthcare Cost and Utilization Project (HCUP). Accessed July 1, 2021. http://hcupnet.ahrq.gov 2. Kaye KS, Patel DA, Stephens JM, Khachatryan A, Patel A, Johnson K. Rising United States hospital admissions for acute bacterial skin and skin structure infections: recent trends and economic impact. PLoS One. 2015;10(11):e0143276. doi:10.1371/journal.pone.0143276 3. Kaye KS, Petty LA, Shorr AF, Zilberberg MD. Current epidemiology, etiology, and burden of acute skin infections in the United States. Clin Infect Dis. 2019;68(suppl 3):S193-S199. doi:10.1093/cid/ciz002 4. Agency for Healthcare Research and Quality. 2018 Data from HCUPnet, Healthcare Cost and Utilization Project (HCUP). Accessed July 1, 2021. http://hcupnet.ahrq.gov 5. Bookstaver PB, Jenkins TC, Stenehjem E, et al. Impact of outpatient vs inpatient ABSSSI treatment on outcomes: a retrospective observational analysis of medical charts across US emergency departments. Open Forum Infect Dis. 2018;5(7):ofy109. doi:10.1093/ofid/ofy109 6. Keyloun KR, Weber DJ, Gardstein BM, Berger A, Gillard P, Ganz ML. Economic burden of hospital admissions for patients with acute bacterial skin and skin structure infections in the United States. Hosp Pract (1995). 2018;46(5):278-286. doi:10.1080/21548331.2018.1506673 7. LaPensee KT, Fan W, Wang Y. Economic burden of hospitalization with antibiotic treatment for ABSSSI in the United States: an analysis of the premier hospital database. Abstract presented at: IDWeek Annual Meeting. October 17-21, 2012; San Diego, CA. Abstract PN22.
* INDICATIONS AND USAGE
- Both KIMYRSA® and ORBACTIV® are oritavancin products that are indicated for the treatment of adult patients with acute bacterial skin and skin structure infections (ABSSSI) caused or suspected to be caused by susceptible isolates of the following gram-positive microorganisms: Staphylococcus aureus (including methicillin-susceptible [MSSA] and methicillin-resistant [MRSA] isolates), Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus dysgalactiae, Streptococcus anginosus group (includes S. anginosus, S. intermedius, and S. constellatus), and Enterococcus faecalis (vancomycin-susceptible isolates only).
- To reduce the development of drug-resistant bacteria and maintain the effectiveness of oritavancin and other antibacterial drugs, oritavancin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria.
- KIMYRSA® and ORBACTIV® are not approved for combination use and have differences in dose strength, duration of infusion, and preparation instructions, including reconstitution and dilution instructions and compatible diluents. Please see the full Prescribing Information for each product.
IMPORTANT SAFETY INFORMATION
Contraindications
- Use of intravenous unfractionated heparin sodium is contraindicated for 120 hours (5 days) after oritavancin administration because the activated partial thromboplastin time (aPTT) test results may remain falsely elevated for approximately 120 hours (5 days) after oritavancin administration.
- Oritavancin products are contraindicated in patients with known hypersensitivity to oritavancin.
Warnings and Precautions
- Coagulation test interference: Oritavancin has been shown to artificially prolong aPTT for up to 120 hours, and may prolong PT and INR for up to 12 hours and ACT for up to 24 hours. Oritavancin has also been shown to elevate D-dimer concentrations up to 72 hours. For patients who require aPTT monitoring within 120 hours of oritavancin dosing, consider a non-phospholipid dependent coagulation test such as a Factor Xa (chromogenic) assay or an alternative anticoagulant not requiring aPTT.
- Serious hypersensitivity reactions, including anaphylaxis, have been reported with the use of oritavancin products. Discontinue infusion if signs of acute hypersensitivity occur. Closely monitor patients with known hypersensitivity to glycopeptides.
- Infusion related reactions: Infusion reactions characterized by chest pain, back pain, chills and tremor have been observed with the use of oritavancin products, including after the administration of more than one dose of oritavancin during a single course of therapy. Stopping or slowing the infusion may result in cessation of these reactions.
- Clostridioides difficile-associated diarrhea: Evaluate patients if diarrhea occurs.
- Concomitant warfarin use: Oritavancin has been shown to artificially prolong PT/INR for up to 12 hours. Patients should be monitored for bleeding if concomitantly receiving oritavancin products and warfarin.
- Osteomyelitis: Institute appropriate alternate antibacterial therapy in patients with confirmed or suspected osteomyelitis.
- Prescribing oritavancin products in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of development of drug-resistant bacteria.
Adverse Reactions
- The most common adverse reactions (≥3%) in patients treated with oritavancin products were headache, nausea, vomiting, limb and subcutaneous abscesses, and diarrhea. The adverse reactions occurring in ≥2 patients receiving KIMYRSA® were hypersensitivity, pruritus, chills and pyrexia.
Please see full Prescribing Information for ORBACTIV®.
Please see full Prescribing Information for KIMYRSA®.
Important Safety InformationINDICATIONS AND USAGE
Both KIMYRSA® and ORBACTIV® are oritavancin products that are indicated for the treatment of adult patients with acute bacterial skin and skin structure infections (ABSSSI) caused or suspected to be
ContraindicationsUse of intravenous unfractionated heparin sodium is contraindicated for 120 hours (5 days) after oritavancin
- Real Patient Results
- SOLO Studies
- Mechanisms
- ABSSSI
- ABSSSI
This site is intended for US Healthcare Professionals only.


