- Treatment
Landscape- ABSSSI
Treatment
Burden- ABSSSI
Treatment
Challenges- About
KIMYRSA®- What is
KIMYRSA®?- Mechanisms
of Action- PK Profile
- In Vitro
Results by Pathogen- Clinical
Studies- Clinical Study Information
- SOLO Studies
- Real-World
Experience- Real-World Studies
- Real Patient Results
- Clinical Expert Videos
- Single-Dose
Administration- Resources
and Support

Real-world reductions in hospitalizations, length of hospital stay, and
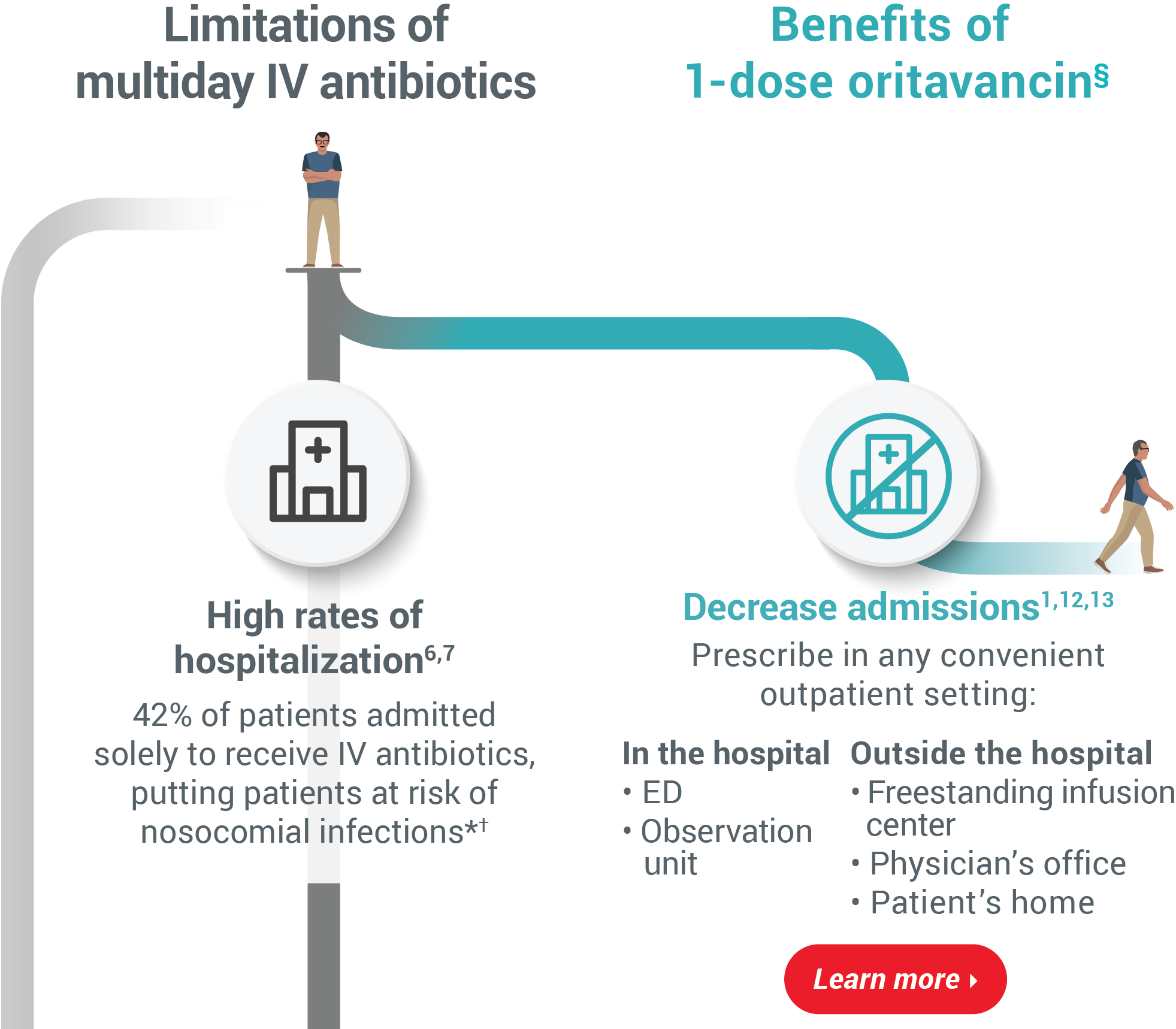
readmissions1-5 Limitations of multiday IV antibioticsBenefits of 1-dose oritavancin§High rates of hospitalization6,7
42% of patients admitted solely to receive
IV antibiotics, putting patients at risk of
nosocomial infections*†Decrease admissions1,12,13
Prescribe in any convenient outpatient setting:In the hospital
• ED
• Observation unitOutside the hospital
• Freestanding infusion center
• Physician’s office
• Patient’s homeProlonged, costly hospital stays2,8-10‡
Vancomycin: 7-10 days8
Daptomycin: 7-14 days9
Inpatient cost per day: ~$2,0002,10Elevated readmission risk11
~12% national 30-day readmission
rate related to skin infectionsReduce readmissions1,3-5
Consistently low infection-related 30-day
readmission rates of 0%-3.6%¶
Learn moreDue to their nature, retrospective studies can contain material limitations and their results should be considered in light of the entire body of available evidence, including clinical trial data. This work was funded by Melinta Therapeutics.
*In a prospective study of 619 patients presenting with serious skin infections.6
†In a 2015 survey of 199 hospitals.7
‡Average length of stay is 5 days.10
§Studies conducted with patients receiving ORBACTIV.1-5,12,13
||Results from a real-world, retrospective, descriptive cohort study of 199 patients.2
¶Results from 4 distinct retrospective studies comprised of a combined 611 patients.1,3-5The efficacy of KIMYRSA® has been established from adequate and well-controlled trials of another oritavancin product, ORBACTIV®, in patients with ABSSSI.
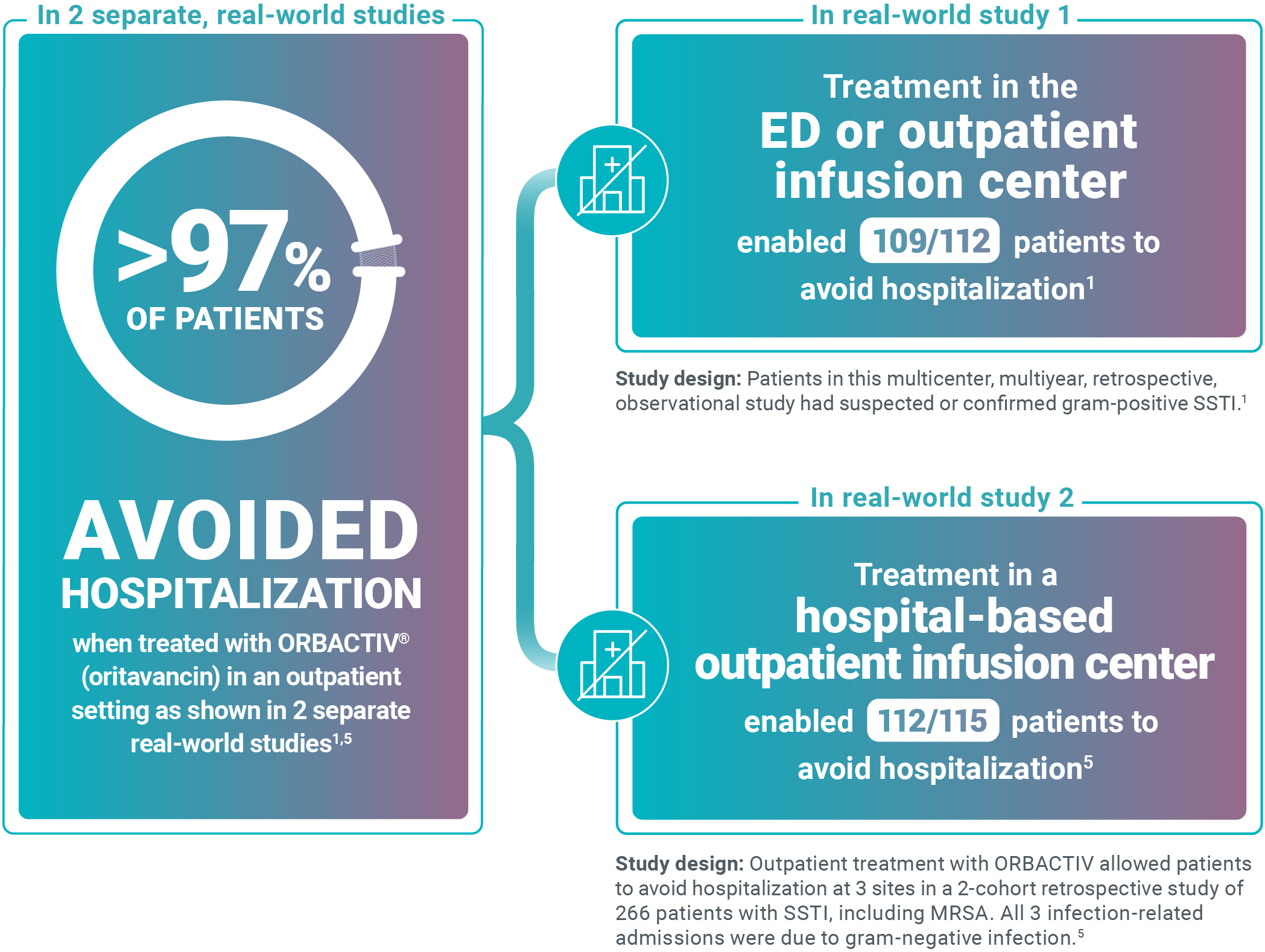
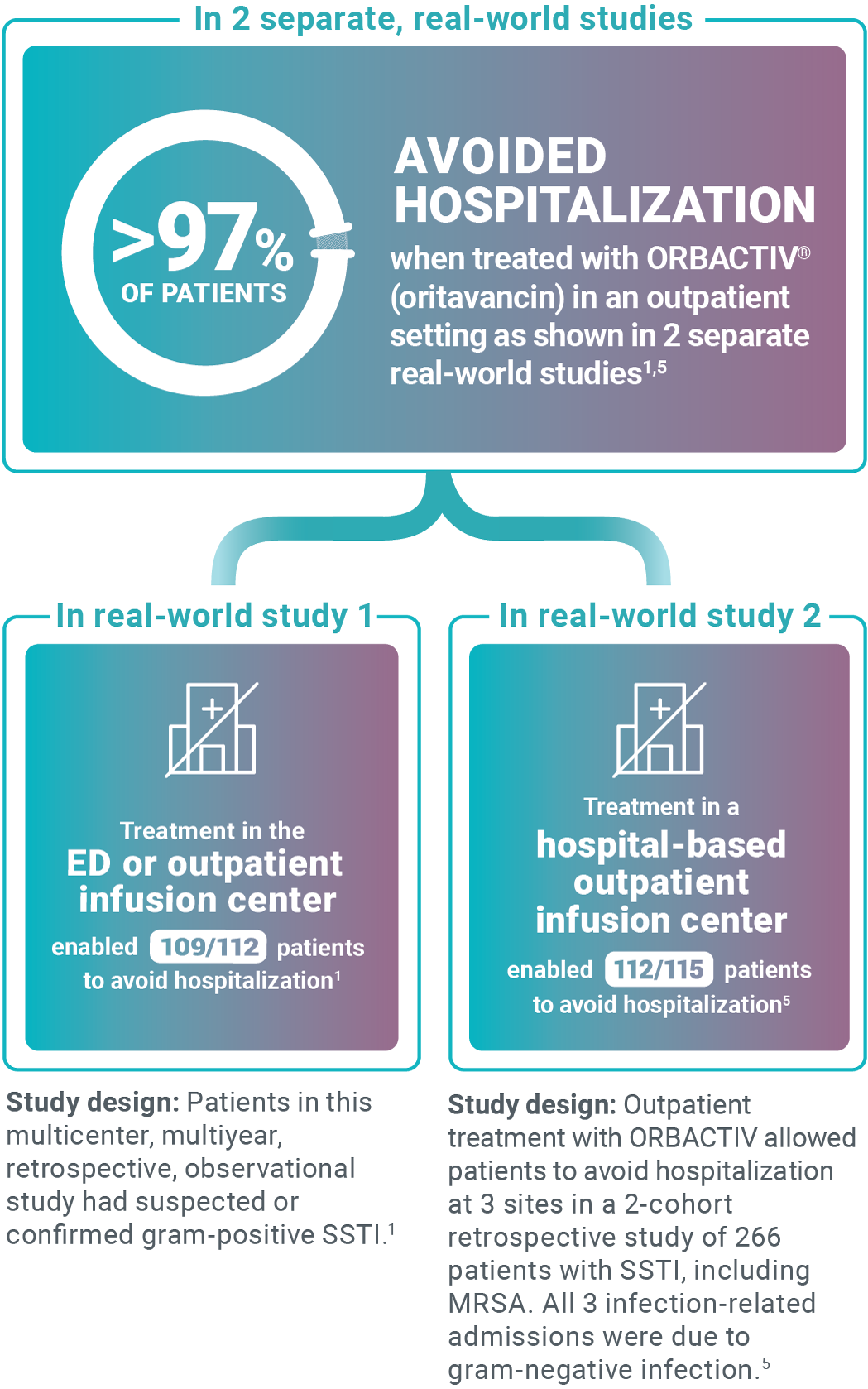
Avoid unnecessary ABSSSI hospitalizations

 Reduce length of stay for ABSSSI patients
Reduce length of stay for ABSSSI patients
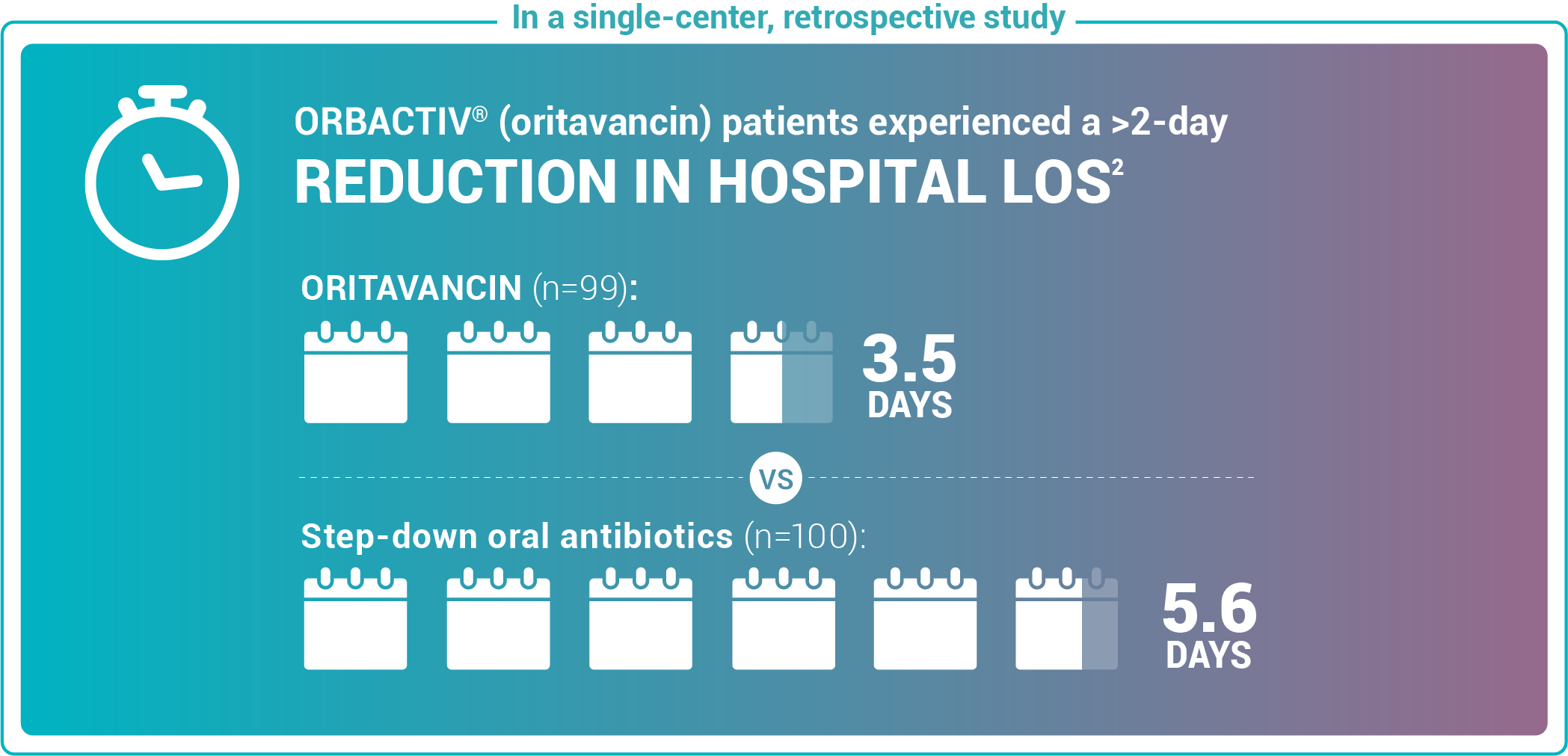
Study design: Admitted patients who received either oritavancin (n=99) to expedite discharge or were discharged on oral step-down antibiotics (n=100).2

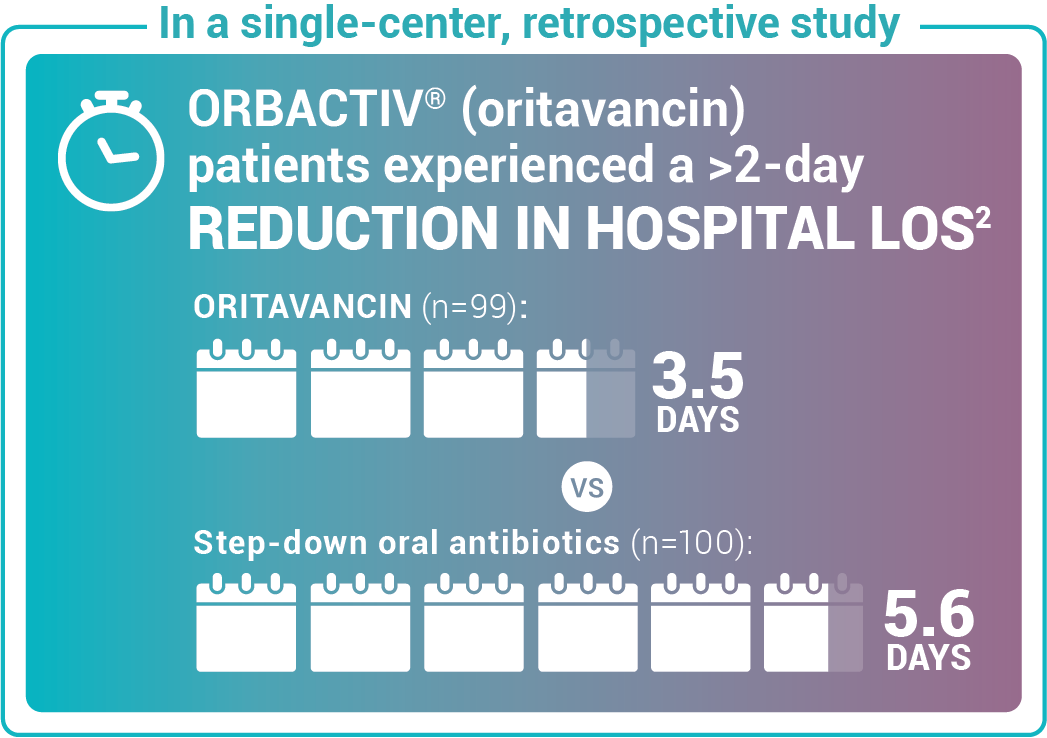
Study design: Admitted patients who received either oritavancin (n=99) to expedite discharge or were discharged on oral step-down antibiotics (n=100).2
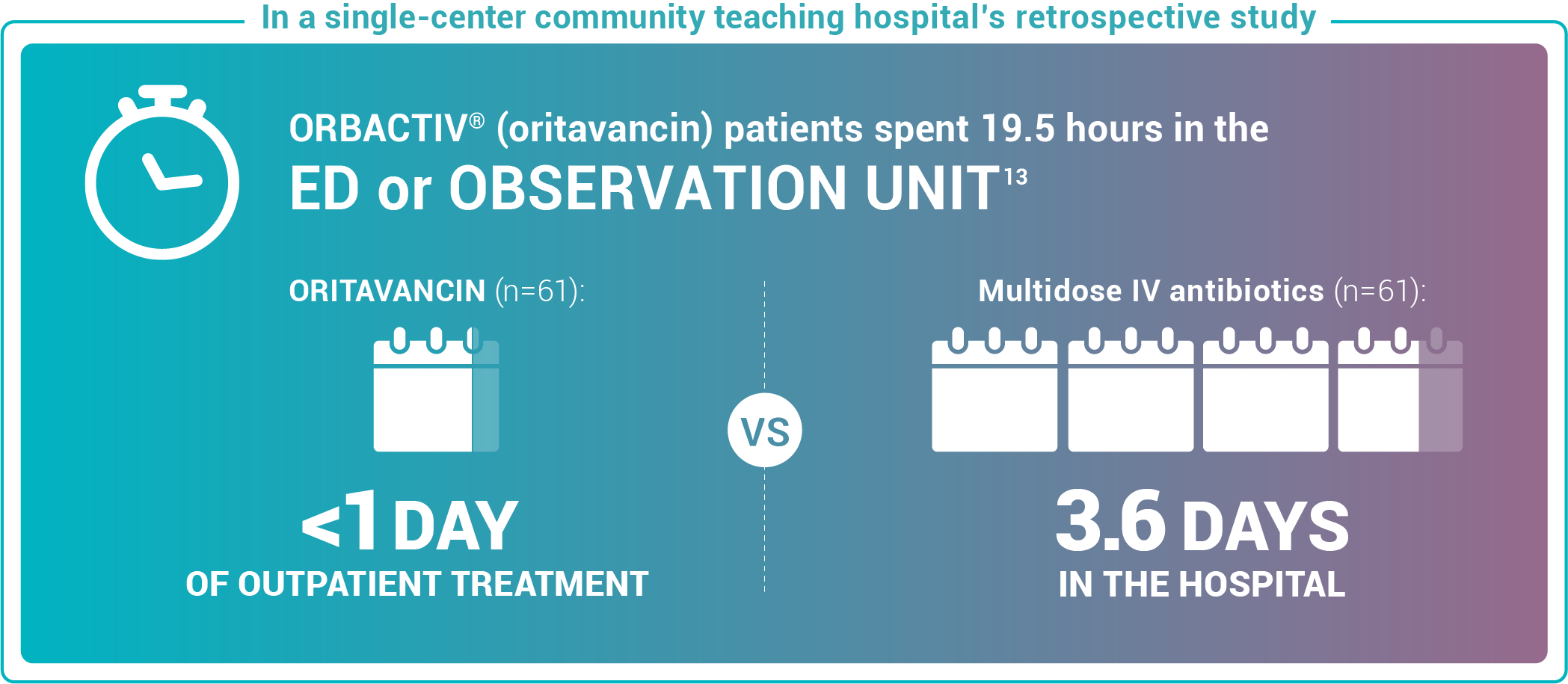
Study design: This pilot evaluated the use of oritavancin in the ED or observation unit as a measure to avert hospital inpatient admissions between January 2017 and December 2017. The primary outcome was LOS, defined as the total time in hours from presentation to the ED until discharge home, including time spent in the observation or inpatient unit.13
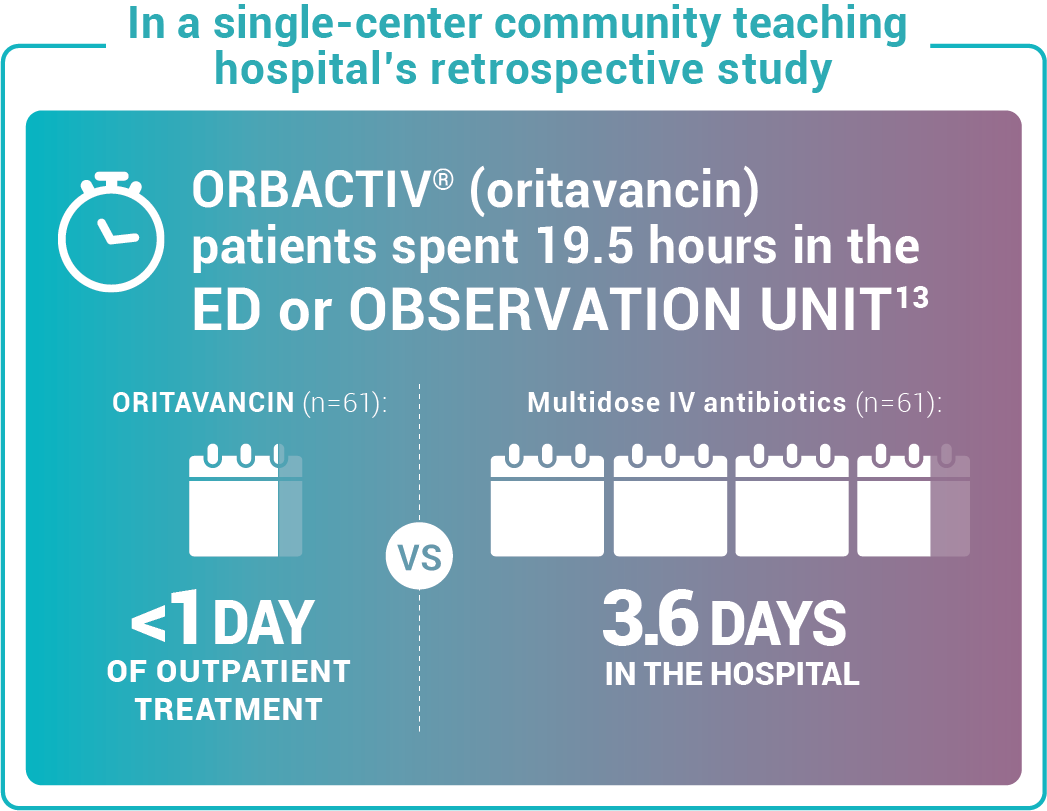
Study design: This pilot evaluated the use of oritavancin in the ED or observation unit as a measure to avert hospital inpatient admissions between January 2017 and December 2017. The primary outcome was LOS, defined as the total time in hours from presentation to the ED until discharge home, including time spent in the observation or inpatient unit.13
Reduce rates of readmissions


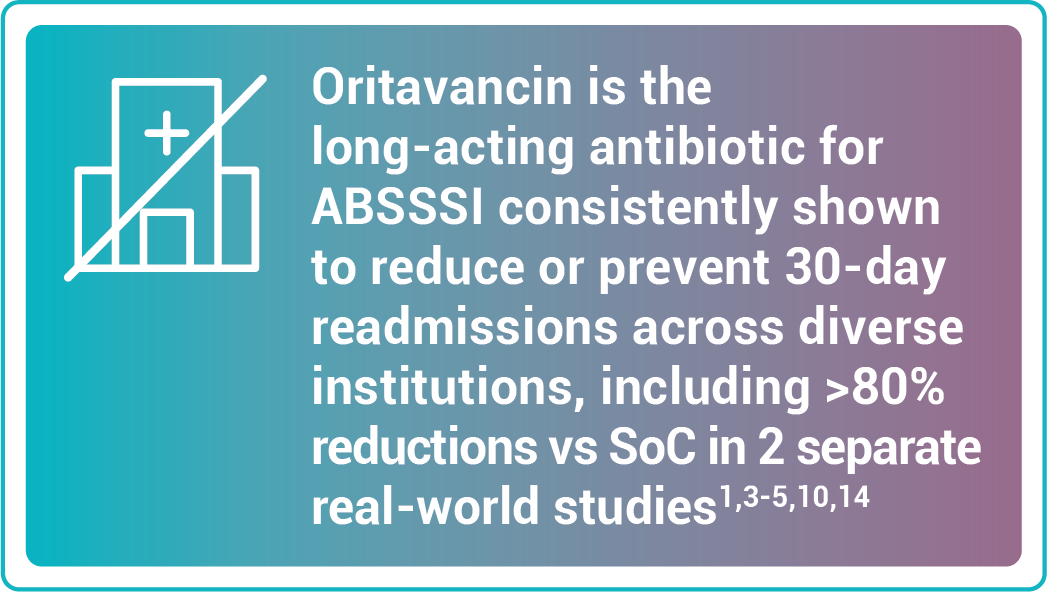
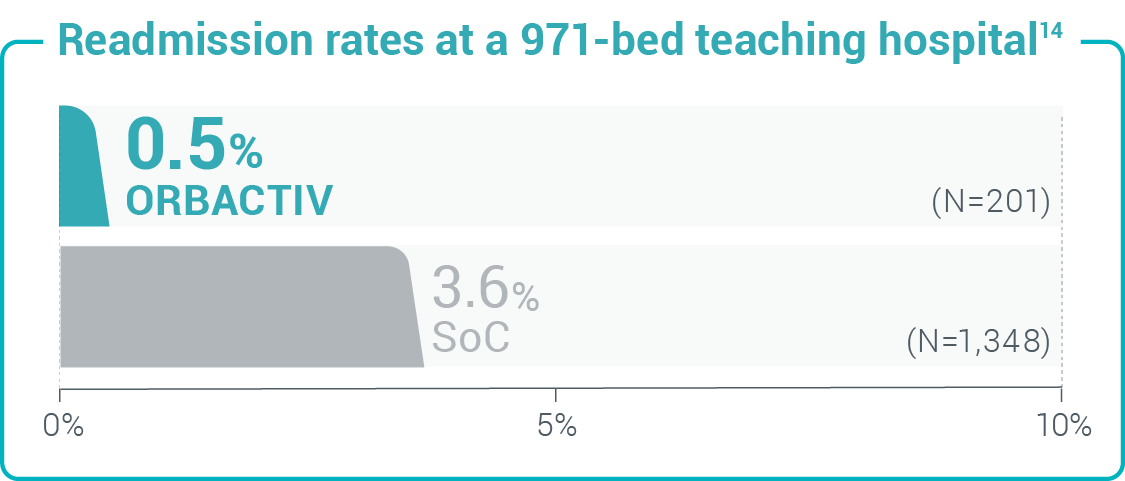 Study design: 30-day readmission or admission rates from a retrospective cohort study of 1,549 patients with acute uncomplicated cellulitis.14
Study design: 30-day readmission or admission rates from a retrospective cohort study of 1,549 patients with acute uncomplicated cellulitis.14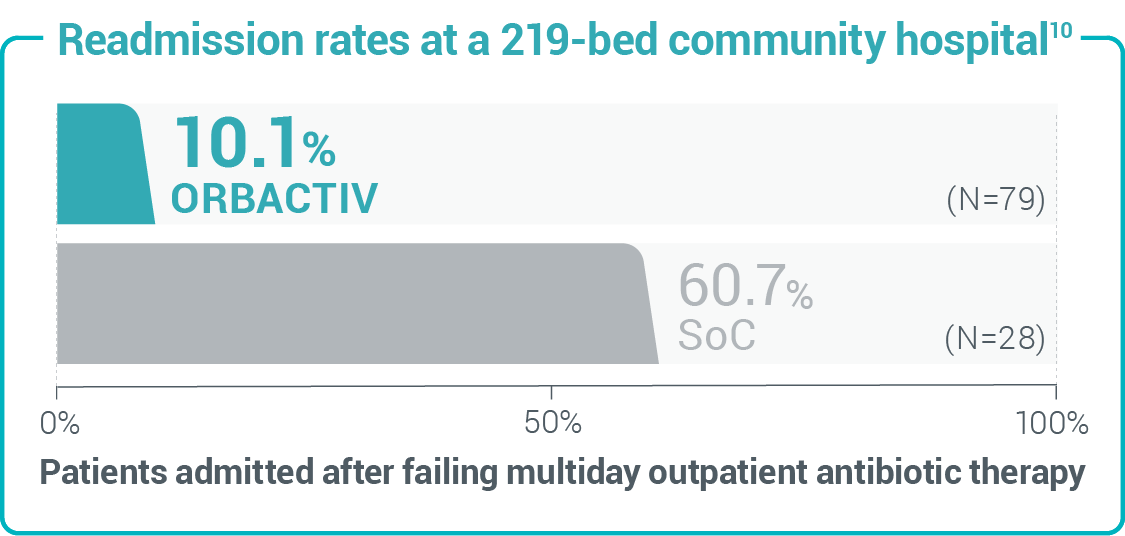 Study design: 30-day readmission rates from a retrospective chart review of 107 patients with cellulitis and abscess.10
Study design: 30-day readmission rates from a retrospective chart review of 107 patients with cellulitis and abscess.10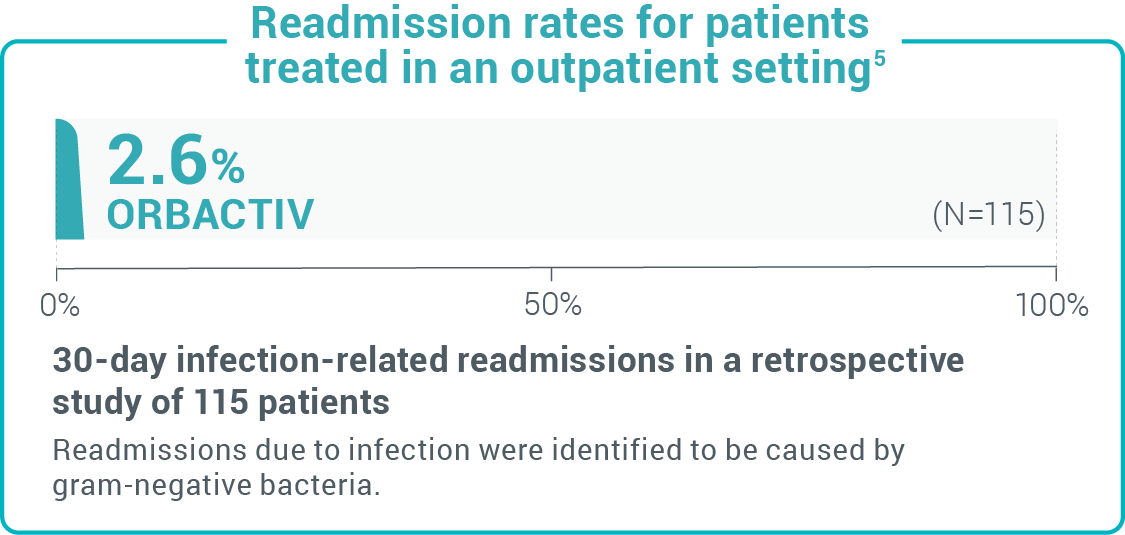 Study design: A retrospective real-world study of readmission rates of patients treated in the outpatient setting with oritavancin for SSTI, including MRSA.5
Study design: A retrospective real-world study of readmission rates of patients treated in the outpatient setting with oritavancin for SSTI, including MRSA.5 Study design: A retrospective observational registry to evaluate the use of, outcomes of, and AEs associated with oritavancin for the treatment of infections presumed or confirmed to be caused by gram-positive bacteria in clinical practice.1
Study design: A retrospective observational registry to evaluate the use of, outcomes of, and AEs associated with oritavancin for the treatment of infections presumed or confirmed to be caused by gram-positive bacteria in clinical practice.1See how 1-dose KIMYRSA can benefit patients
ABSSSI, acute bacterial skin and skin structure infection; ED, emergency department; IV, intravenous; LOS, length of stay; MRSA, methicillin-resistant Staphylococcus aureus; SSTI, skin and soft tissue infections; SoC, standard of care.
References: 1. Redell M, Moeck G, Lucasti C, et al. A real-world patient registry for oritavancin demonstrates efficacy and safety consistent with the phase 3 SOLO program. Open Forum Infect Dis. 2018;5(6):ofy051. doi:10.1093/ofid/ofy051 2. Whittaker C, Lodise TP, Nhan E, Reilly J. Expediting discharge in hospitalized, adult patients with skin and soft tissue infections who received empiric vancomycin therapy with oritavancin: description of findings from an institutional pathway. Drugs Real World Outcomes. 2020;7(suppl 1):30-35. doi:10.1007/s40801-020-00196-6 3. Anastasio PJ, Wolthoff P, Galli A, Fan W. Single-dose oritavancin compared to standard of care IV antibiotics for acute bacterial skin and skin structure infection in the outpatient setting: a retrospective real-world study. Infect Dis Ther. 2017;6(1):115-128. doi:10.1007/s40121-016-0145-7 4. Turner E, Estrada S, Galli A, Armstrong S, Delaportas D. Treatment of acute bacterial skin and skin structure infections (ABSSSI) in the outpatient setting: clinical and economic outcomes from a real-world multi-center study of oritavancin. Poster presented at: ASHP Conference for Pharmacy Leaders; October 17-18, 2016; Chicago, IL. 5. Estrada S, Lodise TP, Tillotson GS, et al. The real world economic and clinical management of adult patients with skin and soft tissue infections (SSTIs) with oritavancin: data from two multicenter observational cohort studies. Drugs Real World Outcomes. 2020;7(suppl 1):6-12. doi:10.1007/s40801-020-00199-3 6. Talan DA, Salhi BA, Moran GJ, et al. Factors associated with decision to hospitalize emergency department patients with skin and soft tissue infection. West J Emerg Med. 2015;16(1):89-97. doi:10.5811/westjem.2014.11.24133 7. Magill SS, O’Leary E, Janelle DL, et al. Changes in prevalence of health care-associated infections in U.S. hospitals. N Engl J Med. 2018;379:1732-44. doi:10.1056/NEJMoa1801550 8. Corey G, Arhin F, Wikler MA, et al. Pooled analysis of single-dose oritavancin in the treatment of acute bacterial skin and skin-structure infections caused by gram-positive pathogens, including a large patient subset with methicillin-resistant Staphylococcus aureus. Int J Antimicrob Agents. 2016;48(5):528-534. doi:10.1016/j.ijantimicag.2016.07.019 9. Daptomycin. Package insert. Merck Sharp & Dohme LLC; 2022. 10. Saddler K, Zhang J, Sul J, et al. Improved economic and clinical outcomes with oritavancin versus a comparator group for treatment of acute bacterial skin and skin structure infections in a community hospital. PLoS One. 2021;16(3):e0248129. doi:10.1371/journal.pone.0248129 11. Bookstaver PB, Jenkins TC, Stenehjem E, et al. Impact of outpatient vs inpatient ABSSSI treatment on outcomes: a retrospective observational analysis of medical charts across US emergency departments. Open Forum Infect Dis. 2018;5(7):ofy109. doi:10.1093/ofid/ofy109 12. Lodise T, Redell M, Armstrong S, Sulham K, Corey G. Efficacy and safety of oritavancin relative to vancomycin for patients with acute bacterial skin and skin structure infections (ABSSSI) in the outpatient setting: results from the SOLO clinical trials. Open Forum Infect Dis. 2017;4(1):ofw274. doi:10.1093/ofid/ofw274 13. Helton B, MacWhinnie A, Minor SB, et al. Early directed oritavancin therapy in the emergency department may lead to hospital avoidance compared to standard treatment for acute bacterial skin and skin structure infections: a real-world retrospective analysis. Drugs Real World Outcomes. 2020;7(suppl 1):20-29. doi:10.1007/ s40801-020-00201-y 14. Williams B, Muklewicz J, Steuber T, Williams A, Edwards J. Comparison of inpatient standard-of-care to outpatient oritavancin therapy for patients with acute uncomplicated cellulitis. J Pharm Pract. 2023;36(1):27-32. doi:10.1177/08971900211021258
*INDICATION AND USAGE
- Both KIMYRSA® and ORBACTIV® are oritavancin products that are indicated for the treatment of adult patients with acute bacterial skin and skin structure infections (ABSSSI) caused or suspected to be caused by susceptible isolates of the following gram-positive microorganisms: Staphylococcus aureus (including methicillin-susceptible [MSSA] and methicillin-resistant [MRSA] isolates), Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus dysgalactiae, Streptococcus anginosus group (includes S. anginosus, S. intermedius, and S. constellatus), and Enterococcus faecalis (vancomycin-susceptible isolates only).
- To reduce the development of drug-resistant bacteria and maintain the effectiveness of oritavancin and other antibacterial drugs, oritavancin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria.
- KIMYRSA® and ORBACTIV® are not approved for combination use and have differences in dose strength, duration of infusion, and preparation instructions, including reconstitution and dilution instructions and compatible diluents. Please see the full Prescribing Information for each product.
IMPORTANT SAFETY INFORMATION
Contraindications
- Use of intravenous unfractionated heparin sodium is contraindicated for 120 hours (5 days) after oritavancin administration because the activated partial thromboplastin time (aPTT) test results may remain falsely elevated for approximately 120 hours (5 days) after oritavancin administration.
- Oritavancin products are contraindicated in patients with known hypersensitivity to oritavancin.
Warnings and Precautions
- Coagulation test interference: Oritavancin has been shown to artificially prolong aPTT for up to 120 hours, and may prolong PT and INR for up to 12 hours and ACT for up to 24 hours. Oritavancin has also been shown to elevate D-dimer concentrations up to 72 hours. For patients who require aPTT monitoring within 120 hours of oritavancin dosing, consider a non-phospholipid dependent coagulation test such as a Factor Xa (chromogenic) assay or an alternative anticoagulant not requiring aPTT.
- Serious hypersensitivity reactions, including anaphylaxis, have been reported with the use of oritavancin products. Discontinue infusion if signs of acute hypersensitivity occur. Closely monitor patients with known hypersensitivity to glycopeptides.
- Infusion related reactions: Infusion reactions characterized by chest pain, back pain, chills and tremor have been observed with the use of oritavancin products, including after the administration of more than one dose of oritavancin during a single course of therapy. Stopping or slowing the infusion may result in cessation of these reactions.
- Clostridioides difficile-associated diarrhea: Evaluate patients if diarrhea occurs.
- Concomitant warfarin use: Oritavancin has been shown to artificially prolong PT/INR for up to 12 hours. Patients should be monitored for bleeding if concomitantly receiving oritavancin products and warfarin.
- Osteomyelitis: Institute appropriate alternate antibacterial therapy in patients with confirmed or suspected osteomyelitis.
- Prescribing oritavancin products in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of development of drug-resistant bacteria.
Adverse Reactions
- The most common adverse reactions (≥3%) in patients treated with oritavancin products were headache, nausea, vomiting, limb and subcutaneous abscesses, and diarrhea. The adverse reactions occurring in ≥2 patients receiving KIMYRSA® were hypersensitivity, pruritus, chills and pyrexia.
Please see Full Prescribing Information for ORBACTIV®.
Please see Full Prescribing Information for KIMYRSA®.
Important Safety Information*INDICATION AND USAGE
Both KIMYRSA® and ORBACTIV® are oritavancin products that are indicated for the treatment of adult patients with acute bacterial skin and skin structure infections (ABSSSI) caused or suspected to be
ContraindicationsUse of intravenous unfractionated heparin sodium is contraindicated for 120 hours (5 days) after oritavancin
- Real Patient Results
- SOLO Studies
- Mechanisms
- ABSSSI
- ABSSSI
This site is intended for US Healthcare Professionals only.