- Treatment
Landscape- ABSSSI
Treatment
Burden- ABSSSI
Treatment
Challenges- About
KIMYRSA®- What is
KIMYRSA®?- Mechanisms
of Action- PK Profile
- In Vitro
Results by Pathogen- Clinical
Studies- Clinical Study Information
- SOLO Studies
- Real-World
Experience- Real-World Studies
- Real Patient Results
- Clinical Expert Videos
- Single-Dose
Administration- Resources
and Support- Patient Support Programs


Real patient results with 1-dose, 1-hour KIMYRSA® for ABSSSI
REBECCA E. ABSSSI PATIENT89-year-old with wound infection avoided hospitalization with KIMYRSA BEFOREadministration of KIMYRSA
BEFOREadministration of KIMYRSA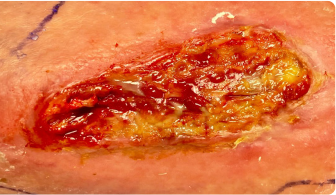 4 WEEKS AFTERadministration of KIMYRSA
4 WEEKS AFTERadministration of KIMYRSA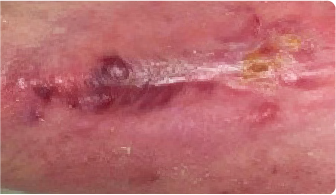 BEFOREadministration of KIMYRSA
BEFOREadministration of KIMYRSA 4 WEEKS AFTERadministration of KIMYRSA
4 WEEKS AFTERadministration of KIMYRSA
This case study is an actual ABSSSI patient who was treated with a single 1200-mg dose of KIMYRSA. Individual treatment results may vary.
This case study is an actual ABSSSI patient who was treated with a single 1200-mg dose of KIMYRSA. Individual treatment results may vary.
Background and Presentation
 89-year-old female with history of chronic ulceration to the right anterior tibia presented to physician’s office with lower right extremity wound infection
89-year-old female with history of chronic ulceration to the right anterior tibia presented to physician’s office with lower right extremity wound infectionCase Details
- Patient’s infection began following melanoma excision and subsequent radiation on the lower right extremity
- Wound extended to soft tissue structures including anterior tibial tendon; MRI confirmed no bone involvement
- Patient currently resides in assisted-living facility, and her son is also very active in her healthcare
Comorbidities and health considerations
Patient presented with the following conditions (all well managed with medication):- Diabetes mellitus
- Hypertension
- Hypothyroidism
- Chronic atrial fibrillation requiring anticoagulation therapy
- Stage Illa kidney disease
Current medications:
- Albuterol
- Apixaban
- Atenolol
- Cetirizine
- Dulaglutide
- Ferrous sulfate
- Furosemide
- Levothyroxine
- Nystatin
- Pravastatin
- Pregabalin
- Tramadol
- Triamcinolone
Prior surgeries include:
- Total knee replacement
- Shoulder, cervical spine, and other orthopedic surgeries
- Melanoma excision from right leg
Evaluation details
- BP: 128/77
- HbAlc: 6.2%
- BMI: 35
- WBC: 12.24
- History of cellulitis, incompletely resolved on oral antibiotics
- Infection site culture pathogens: Group B Streptococcus and MRSA
- ESR: 66 mm/hr
- ANA: Positive
- Temp: 36.7 °C (97.9 °F)
- Pulse: 77 bpm
Diagnosis
 Wound infection positive for Group B Streptococcus and MRSA, Eron class II
Wound infection positive for Group B Streptococcus and MRSA, Eron class IITreatment course

- Multiple rounds of doxycycline and linezolid with poor tolerance and limited efficacy
- Wound debridement with deep cultures following poor response to doxycycline and linezolid
- Patient and her son wished to avoid hospitalization and patient was at risk for worsening renal function with other antibiotics, including vancomycin
- Treated post-operatively with single-dose KIMYRSA, which was well tolerated upon administration at hospital outpatient infusion center
- $0 out-of-pocket cost to the patient with Medicare plus Medigap coverage
Individual insurance coverage and out-of-pocket costs may vary.
KIMYRSA results
 Early clinical response followed by cure at 4 weeks after KIMYRSA® (oritavancin)
Early clinical response followed by cure at 4 weeks after KIMYRSA® (oritavancin)BEFORE
administration of KIMYRSA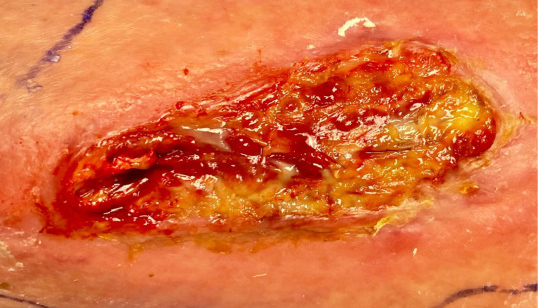
48 HOURS AFTER
administration of KIMYRSA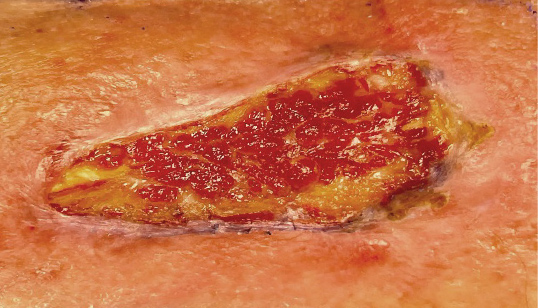
7 DAYS AFTER
administration of KIMYRSA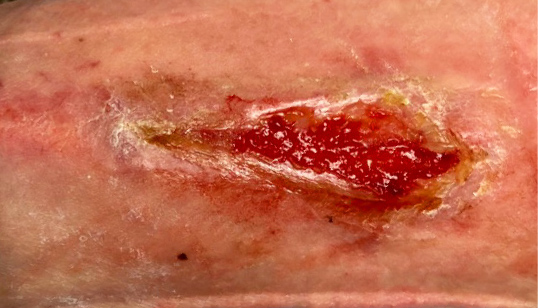
4 WEEKS AFTER
administration of KIMYRSA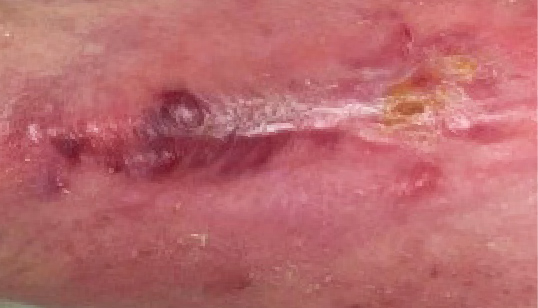
Quotes
“Risk of hospital-acquired infection and prolonged immobility would certainly have put this patient at risk in a hospital setting. We were able to avoid the pitfalls of hospitalization and heal a very challenging wound thanks to KIMYRSA, weekly local wound care, and a good son. A great outcome for all those involved!”
-Allen Raphael, DPM, FACFAS*
“Access to outpatient antibiotic therapy is absolutely ideal for my mother. Her recent hospital stays have resulted in additional infections and illnesses, and set her back physically and mentally. Having the opportunity to receive necessary therapy with KIMYRSA in an outpatient setting greatly reduced the health risks to her, and the strain on our family.”
-Patient’s son
*The treating physician is a paid consultant of Melinta Therapeutics, LLC.
RANDOLPH B. ABSSSI PATIENT68-year-old with cellulitis discharged with KIMYRSA BEFOREadministration of KIMYRSA
BEFOREadministration of KIMYRSA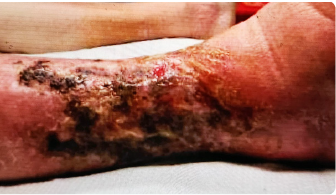 10 WEEKS AFTERadministration of KIMYRSA
10 WEEKS AFTERadministration of KIMYRSA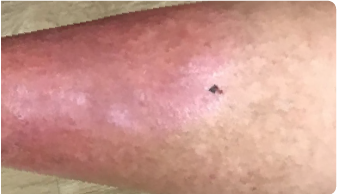 BEFOREadministration of KIMYRSA
BEFOREadministration of KIMYRSA 10 WEEKS AFTERadministration of KIMYRSA
10 WEEKS AFTERadministration of KIMYRSA
This case study is an actual ABSSSI patient who was treated with a single 1200-mg dose of KIMYRSA. The infection photos are those of the patient; however, the patient portrait is not of the actual individual. Individual treatment results may vary.
Background and Presentation
 68-year-old male presented to ED with lower right extremity wound marked by erythema, superficial sloughing, and purulent drainage with areas of crusting and scattered eschar
68-year-old male presented to ED with lower right extremity wound marked by erythema, superficial sloughing, and purulent drainage with areas of crusting and scattered escharCase Details
- Patient reports he accidentally scraped his leg on a bucket ~3 weeks prior to presentation
- Topical antibiotic spray failed to alleviate progressive swelling, pain, erythema, and drainage
- Infection progressed despite 2 days of outpatient cephalexin given by PCP
- Admitted to hospital with lower right extremity cellulitis with purulent drainage
Comorbidities and health considerations
Patient presented with the following conditions (all well managed with medication):- Congestive heart failure
- Gout
- Hypertension
- Atrial fibrillation for which he relies on a pacemaker and anticoagulation therapy
- Stage Illa chronic kidney disease
- Obesity
Current medications:
- Cholecalciferol
- Doxazosin
- Ferrous sulfate
- Levothyroxine
- Metoprolol tartrate
- Rivaroxaban
Evaluation details
- BP: 111/63
- Glucose: 180 mg/dL
- Weight: 115.7 kg (255 Ib)
- WBC: 11.8 (81%N)
- Infection site culture pathogens: presumed group A Streptococcus
- Blood culture pathogens NGTD
- Temp: 36.3 °C (97.4 °F)
- Pulse: 94 bpm
- Cr: 1.55 mg/dL
- eGFR: 48
Diagnosis
 Cellulitis caused by presumed group A Streptococcus, Eron class II
Cellulitis caused by presumed group A Streptococcus, Eron class IITreatment course

- Initial course of inpatient ampicillin/sulbactam and vancomycin, yielding some improvement in erythema and reduced drainage, but without complete resolution of infection
- Discharged with a prescription for outpatient KIMYRSA, which was well tolerated upon administration in physician’s office
- $0 out-of-pocket cost to the patient with Medicare Part B plus Medigap coverage (deductible already met)
Individual insurance coverage and out-of-pocket costs may vary.
KIMYRSA results
 Early clinical response followed by cure at 4 weeks after KIMYRSA® (oritavancin)
Early clinical response followed by cure at 4 weeks after KIMYRSA® (oritavancin)BEFORE
administration of KIMYRSA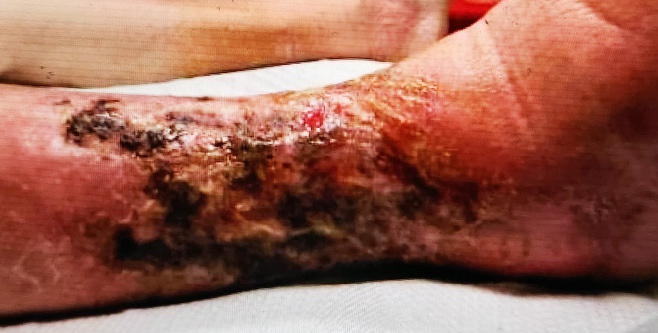
3 WEEKS AFTER
administration of KIMYRSA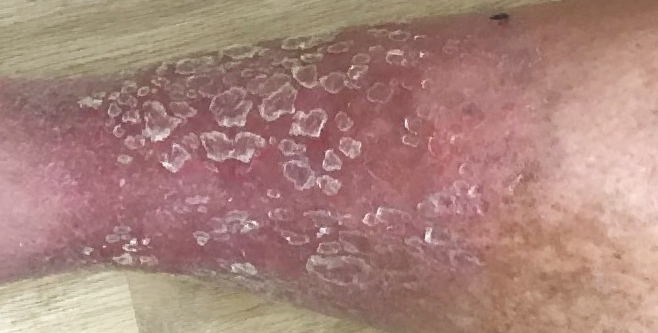
10 WEEKS AFTER
administration of KIMYRSA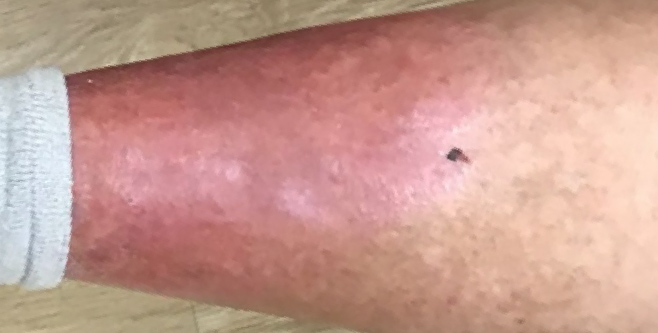
Quotes
“I wanted to treat Randolph with an IV antibiotic, but I did not want to subject him to a PICC line. I was most comfortable prescribing one dose of KIMYRSA because I knew it would provide efficacy, safety, and convenience—and that is exactly what it did.”
-Andrew Dold, DO*
“My infection was really horrible. It kept draining so much that I couldn’t go to church like I wanted to because my sock and shoe would be soaked. When I went to the hospital, they said they had never seen an infection so bad. I was scheduled to see Dr. Dold when I left the hospital. He gave me KIMYRSA and it really helped a lot. My infection totally cleared up and within a month, it was history. It’s a real blessing to be able to get back to doing things I want to do.”
-Randolph B., patient
*The treating physician is a paid consultant of Melinta Therapeutics, LLC.
Help patients get back to their days
*INDICATION AND USAGE
- Both KIMYRSA® and ORBACTIV® are oritavancin products that are indicated for the treatment of adult patients with acute bacterial skin and skin structure infections (ABSSSI) caused or suspected to be caused by susceptible isolates of the following gram-positive microorganisms: Staphylococcus aureus (including methicillin-susceptible [MSSA] and methicillin-resistant [MRSA] isolates), Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus dysgalactiae, Streptococcus anginosus group (includes S. anginosus, S. intermedius, and S. constellatus), and Enterococcus faecalis (vancomycin-susceptible isolates only).
- To reduce the development of drug-resistant bacteria and maintain the effectiveness of oritavancin and other antibacterial drugs, oritavancin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria.
- KIMYRSA® and ORBACTIV® are not approved for combination use and have differences in dose strength, duration of infusion, and preparation instructions, including reconstitution and dilution instructions and compatible diluents. Please see the full Prescribing Information for each product.
IMPORTANT SAFETY INFORMATION
Contraindications
- Use of intravenous unfractionated heparin sodium is contraindicated for 120 hours (5 days) after oritavancin administration because the activated partial thromboplastin time (aPTT) test results may remain falsely elevated for approximately 120 hours (5 days) after oritavancin administration.
- Oritavancin products are contraindicated in patients with known hypersensitivity to oritavancin.
Warnings and Precautions
- Coagulation test interference: Oritavancin has been shown to artificially prolong aPTT for up to 120 hours, and may prolong PT and INR for up to 12 hours and ACT for up to 24 hours. Oritavancin has also been shown to elevate D-dimer concentrations up to 72 hours. For patients who require aPTT monitoring within 120 hours of oritavancin dosing, consider a non-phospholipid dependent coagulation test such as a Factor Xa (chromogenic) assay or an alternative anticoagulant not requiring aPTT.
- Serious hypersensitivity reactions, including anaphylaxis, have been reported with the use of oritavancin products. Discontinue infusion if signs of acute hypersensitivity occur. Closely monitor patients with known hypersensitivity to glycopeptides.
- Infusion related reactions: Infusion reactions characterized by chest pain, back pain, chills and tremor have been observed with the use of oritavancin products, including after the administration of more than one dose of oritavancin during a single course of therapy. Stopping or slowing the infusion may result in cessation of these reactions.
- Clostridioides difficile-associated diarrhea: Evaluate patients if diarrhea occurs.
- Concomitant warfarin use: Oritavancin has been shown to artificially prolong PT/INR for up to 12 hours. Patients should be monitored for bleeding if concomitantly receiving oritavancin products and warfarin.
- Osteomyelitis: Institute appropriate alternate antibacterial therapy in patients with confirmed or suspected osteomyelitis.
- Prescribing oritavancin products in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of development of drug-resistant bacteria.
Adverse Reactions
- The most common adverse reactions (≥3%) in patients treated with oritavancin products were headache, nausea, vomiting, limb and subcutaneous abscesses, and diarrhea. The adverse reactions occurring in ≥2 patients receiving KIMYRSA® were hypersensitivity, pruritus, chills and pyrexia.
Please see Full Prescribing Information for ORBACTIV®.
Please see Full Prescribing Information for KIMYRSA®.
Important Safety Information*INDICATION AND USAGE
Both KIMYRSA® and ORBACTIV® are oritavancin products that are indicated for the treatment of adult patients with acute bacterial skin and skin structure infections (ABSSSI) caused or suspected to be
ContraindicationsUse of intravenous unfractionated heparin sodium is contraindicated for 120 hours (5 days) after oritavancin
- Real Patient Results
- SOLO Studies
- Mechanisms
- ABSSSI
- ABSSSI
This site is intended for US Healthcare Professionals only.


